Il 23 Inhibitor
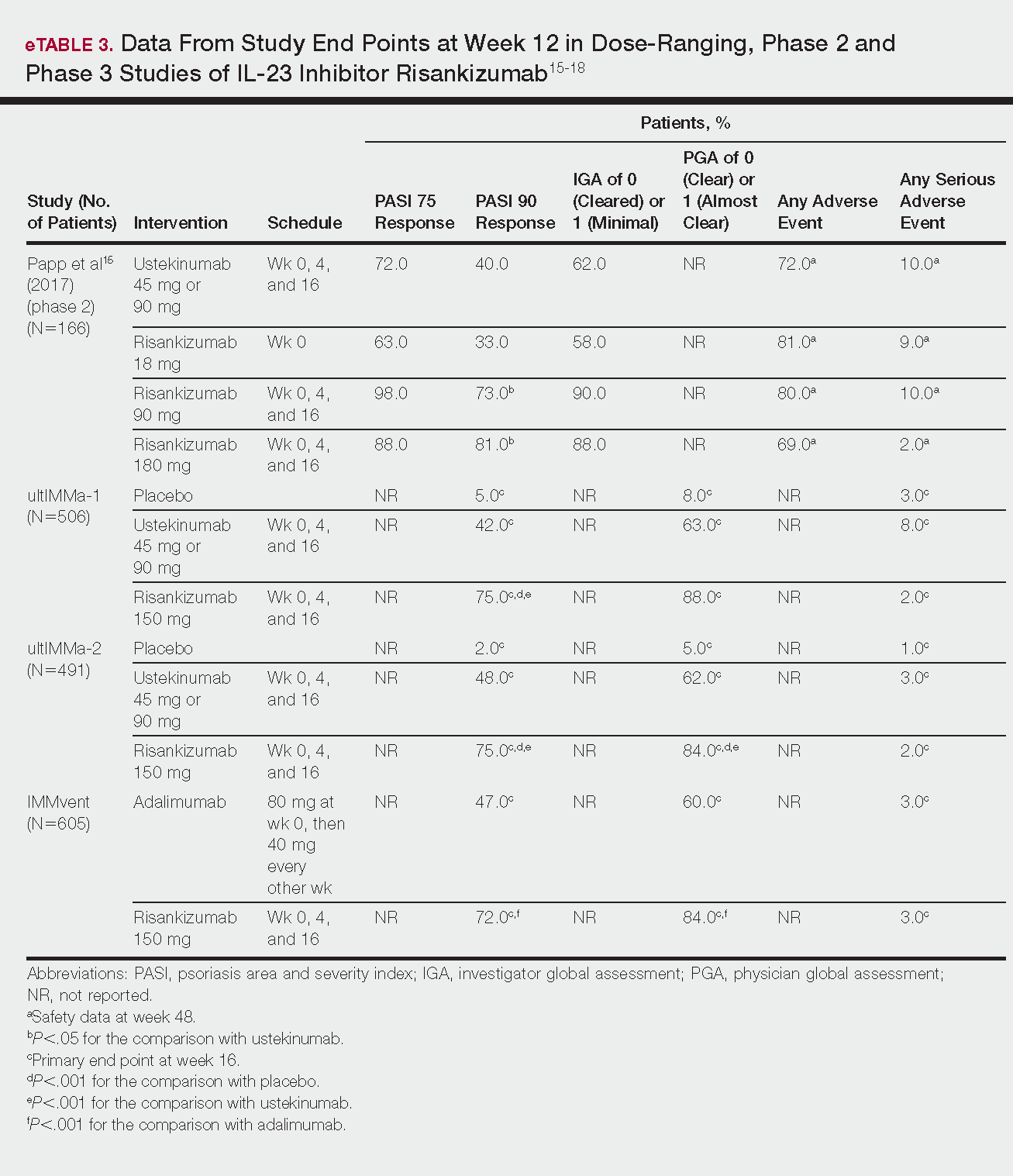
Emerging Therapies In Psoriasis A Systematic Review Mdedge Dermatology

Akt Activity Modulates Th17 Development A Il 17 Expression In T Cells Download Scientific Diagram
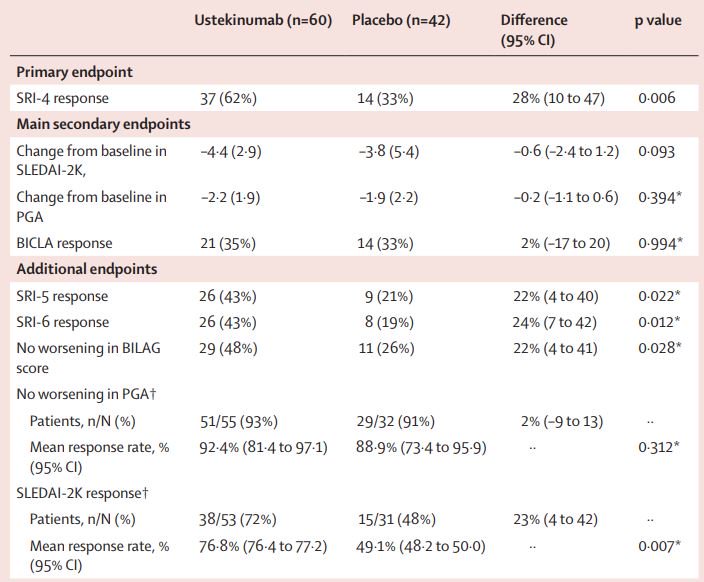
The Lancet Efficacy And Safety Of Ustekinumab An Il 12 And Il 23 Inhibitor In Patients With Active Systemic Lupus Erythematosus Results Of A Multicentre Double Blind Phase 2 Randomised Controlled Study Lancetrheumatology
Http Web Brrh Com Msl Im18 Day 2 Saturday Saturday 1 newer biologics Pdf

Au 12 4869 B2 Modulators Of Il 12 And Or Il 23 For The Prevention Or Treatment Of Alzheimer S Disease The Lens Free Open Patent And Scholarly Search
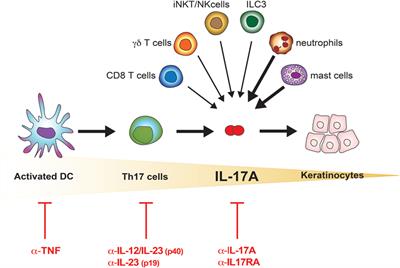
Frontiers The Il 17 Family Of Cytokines In Psoriasis Il 17a And Beyond Immunology
Ilumya marks the second interleukin-23 (IL-23) inhibitor to gain FDA approval within the past year, following the approval of J&J’s Tremfya (guselkumab) in July 17.

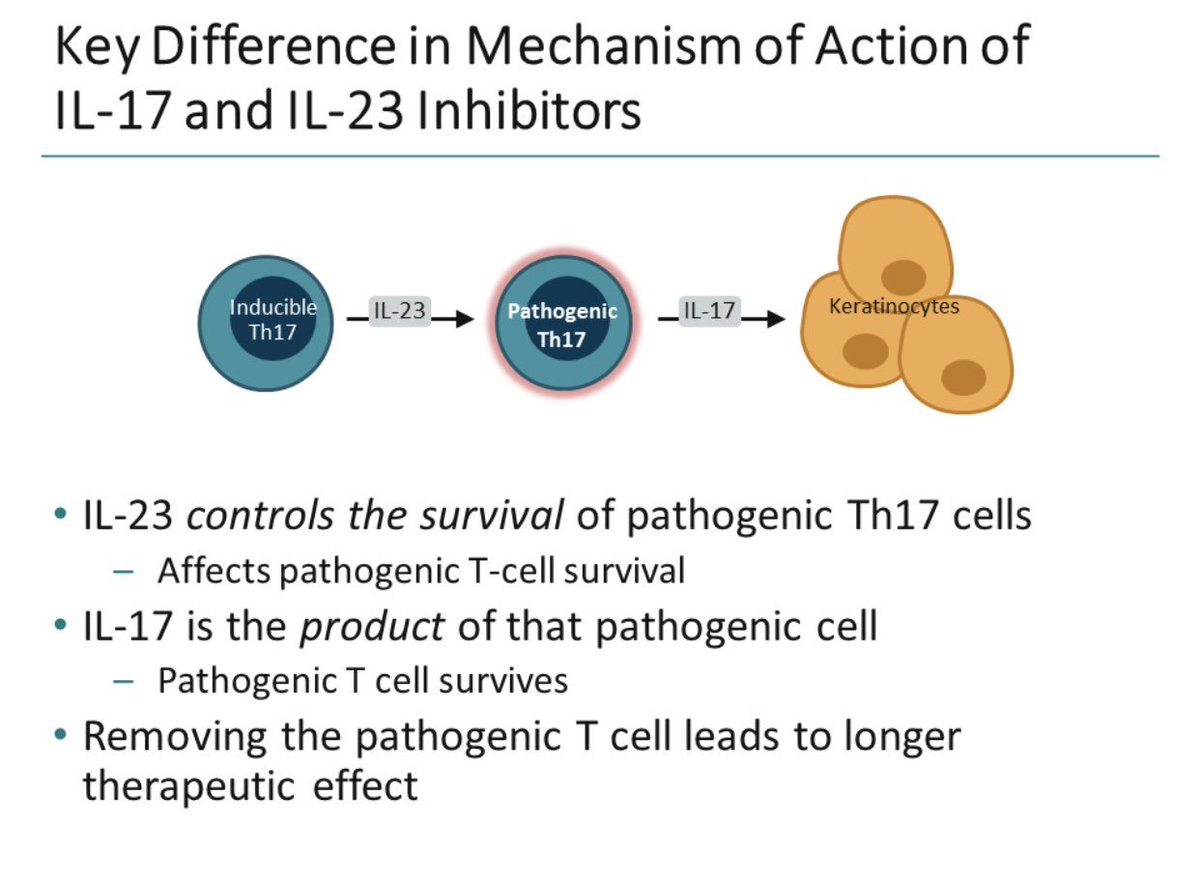
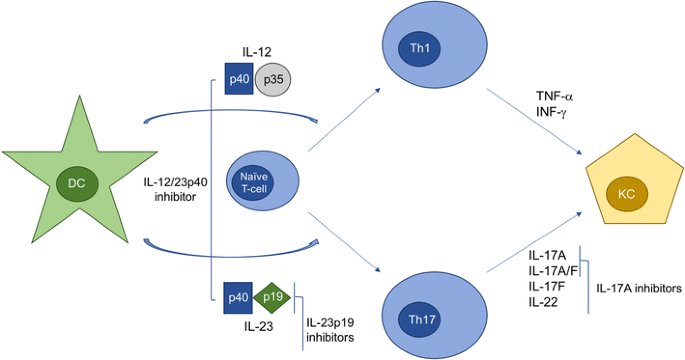
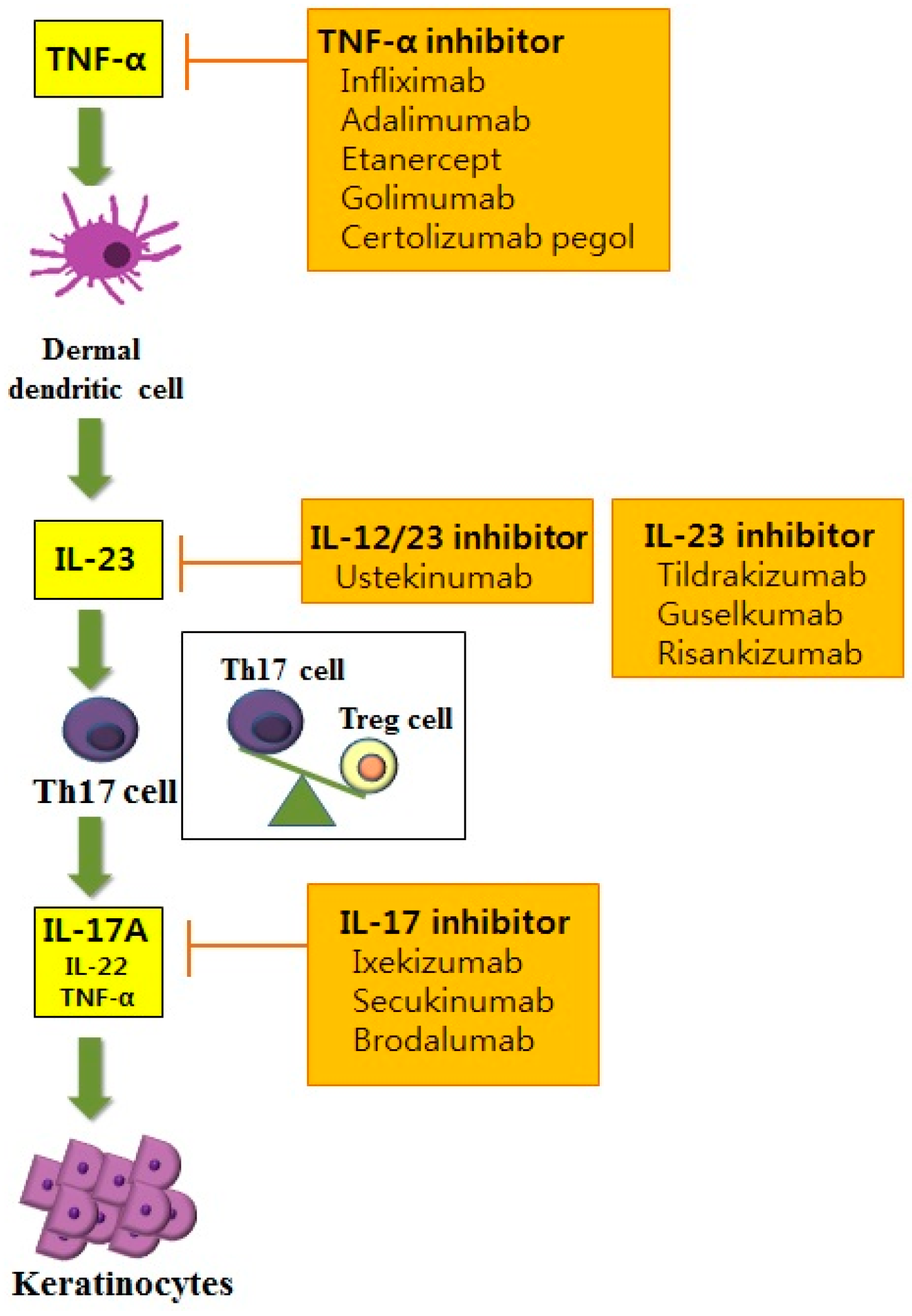
Il 23 inhibitor. Efficacy and safety of induction therapy with the selective IL-23 inhibitor risankizumab (BI ), in patients with moderate-to-severe Crohn’s disease:. Efficacy and safety of ustekinumab, an IL-12 and IL-23 inhibitor, in patients with active systemic lupus erythematosus:. IL-23 is produced by dendritic cells and keratinocytes, among others, causing the proliferation and survival of Th17 cells, as well as the production of IL-17A and IL-22, which are key drivers of the keratinocyte proliferation central to psoriasis.
April 25, 16 IL-12, IL-23 Inhibitor More Effective Than TNF Inhibitors in Psoriasis HealthDay News – Ustekinumab was more effective than tumor necrosis factor-α inhibitors for the treatment of psoriasis at 6 and 12 months, according to a study published in the May issue of the Journal of the American Academy of Dermatology. Efficacy and safety of induction therapy with the selective IL-23 inhibitor risankizumab (BI ), in patients with moderate-to-severe Crohn's disease:. They function especially in regulation of the immune system.
SKYRIZI is an interleukin-23 (IL-23) inhibitor that selectively blocks IL-23 by binding to its p19 subunit. USE USE for SKYRIZI® (risankizumab-rzaa). SARS-CoV-2 infection in a psoriatic patient treated with IL-23 inhibitor Since December 19, an outbreak of 19 novel coronavirus disease (COVID‐19) caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has been spreading worldwide.
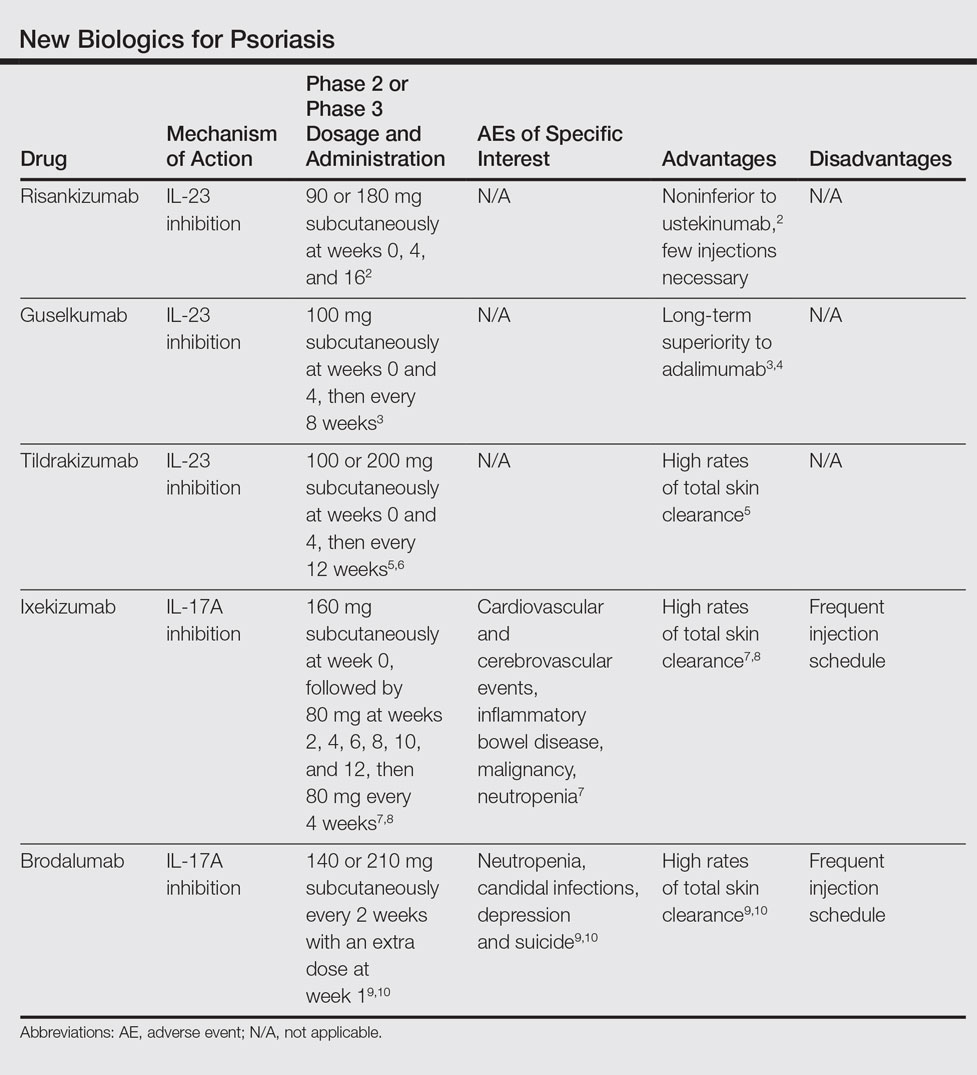
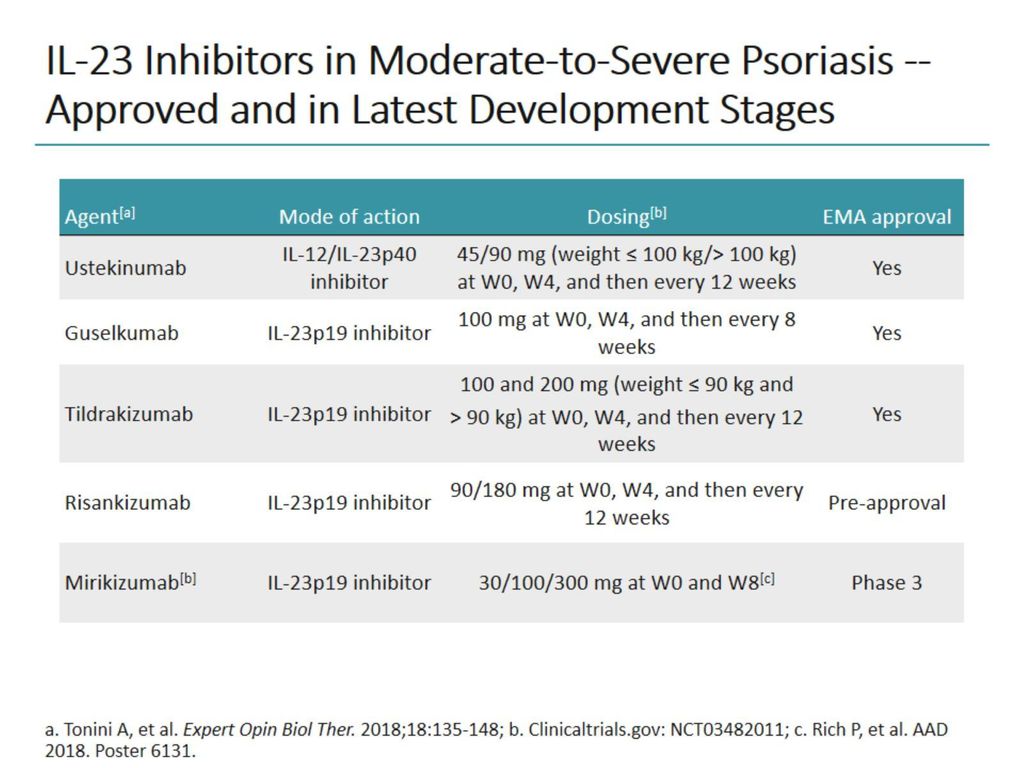
Ustekinumab is a monoclonal antibody targeting interleukin (IL)-12 and IL-23 and is approved for the treatment of plaque psoriasis, psoriatic arthritis, and Crohn's disease. Risankizumab, guselkumab, and tildrakizumab are new IL-23 inhibitors currently in phase 3 trials with promising early efficacy and safety results. Vs PBO in 1,1 adults with active PsA.
This cytokine is linked with inflammation in psoriasis and PsA. Risankizumab (BI /ABBV-066) is a humanised, immunoglobulin G1 monoclonal antibody that selectively inhibits IL-23 by specifically targeting the p19 subunit18and has shown efficacy in psoriasis, psoriatic arthritis (PsA) and Crohn’s disease.19–22This proof-of-concept, dose-ranging study assessed the efficacy and safety of risankizumab in patients with active AS. Janssen’s Tremfya (guselkumab) Receives the US FDA’s Approval as the First Selective IL-23 Inhibitor for Active Psoriatic Arthritis.
Additionally, participants treated with the IL-23 inhibitor experienced improvements in skin manifestations of psoriasis, physical function, enthesitis and dactylitis, and fatigue. A multicentre, 12-week randomised trial. A report on a patient with COVID-19 with psoriatic arthritis receiving ustekinumab, Annals of the Rheumatic Diseases, 10.1136/annrheumdis--, (annrheumdis-.
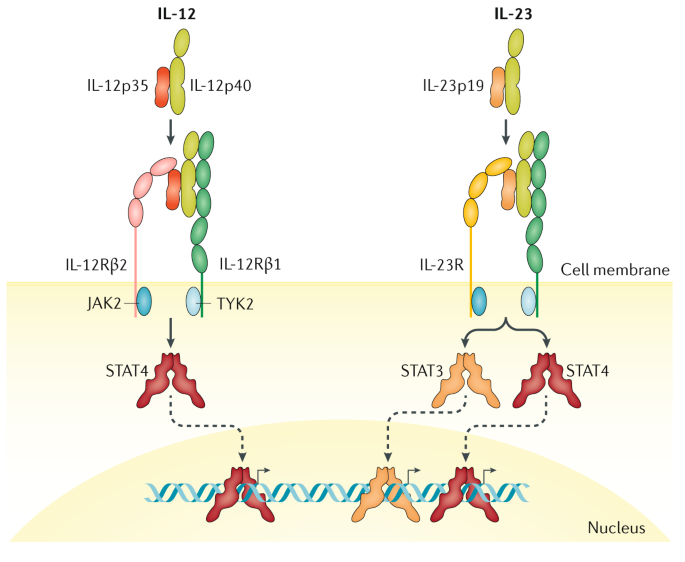
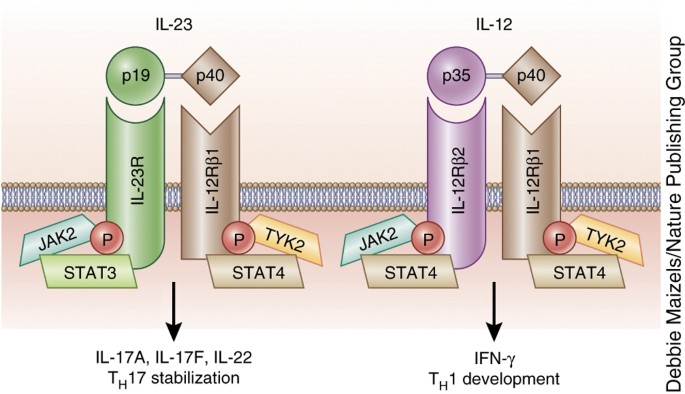
Interleukin-12 (IL-12) and IL-23 promote cellular responses mediated by T cells, which contribute to an inflammatory loop responsible for the induction and maintenance of psoriatic plaques. IL-23 inhibitors target a specific subunit of IL-23. Interleukin-23 (IL-23) is a heterodimeric cytokine composed of an IL12B (IL-12p40) subunit (that is shared with IL12) and the IL23A (IL-23p19) subunit.
Janssen Reports sBLA Submission to the US FDA for Darzalex Faspro (daratumumab and hyaluronidase-fihj) to Treat Patients with Light Chain (AL) Amyloidosis. Some FDA-approved IL-23 inhibitors are:. SKYRIZI is a prescription medicine used to treat adults with moderate to severe plaque psoriasis who may benefit from taking injections or pills (systemic therapy) or treatment using ultraviolet or UV light (phototherapy).
IL-23 has emerged as an important inflammatory cytokine in the pathogenesis of psoriasis. First, for a rheumatologist, combining cytokines like interleukin-1 (IL-1), IL-6, IL-17 and IL-23 into one group is the equivalent of treating chicken, pork and beef the same. Janssen’s Tremfya (guselkumab) Receives the US FDA’s Approval as the First Selective IL-23 Inhibitor for Active Psoriatic Arthritis Shots:.
Overall, the safety profile of guselkumab for the treatment of psoriatic arthritis is generally consistent with that seen in the treatment of plaque psoriasis. Interleukin-23 (IL-23) Inhibitor Clinical Assessment of products The report comprises of comparative clinical assessment of products by development stage, product type, route of administration, molecule type, and MOA type across this mechanism of action. While ustekinumab has demonstrated significant efficacy and.
Interleukin (IL)-23 is present at increased levels in people with plaque psoriasis. The assessment part of the report embraces, in-depth Interleukin-23 (IL-23) Inhibitor commercial assessment and clinical assessment of the pipeline products under. The protein encoded by this gene is a subunit of the receptor for IL-23.This protein pairs with the receptor molecule IL-12Rβ1 (), together forming the IL-23 receptor complex, and both are required for IL-23 signaling.This protein associates constitutively with Janus kinase 2 (), and also binds to transcription activator STAT3 in a ligand-dependent manner.
Francesco Messina, Francesca Pampaloni, Stefano Piaserico, Comment on:. B Feagan et al. Click here to read more about IL-23 inhibitors.
Results of a randomized. IL-23 is part of IL-12 family of cytokines. Although the current data does not provide insight into the long-term effects of these drugs, results have been extremely encouraging.
At a July 18 public meeting of the New England Comparative Effectiveness Public Advisory Council, the group voted that, compared with anti–tumor necrosis factor (anti-TNF) drugs, both guselkumab and risankizumab offered a superior benefit based on currently available data. Research indicates that targeted IL-23 inhibition may be an important step in achieving that goal. 6 Ustekinumab targets the p40 subunit, common to both IL-12 and IL-23, thus blocking the activity of both cytokines.
At a July 18 public meeting of the New England Comparative Effectiveness Public Advisory Council, the group voted that, compared with anti—tumor necrosis factor (anti-TNF) drugs, both guselkumab and risankizumab offered a superior benefit based on currently available data. IL-12 and IL-23 have been implicated in systemic lupus erythematosus. Although the current data does not provide insight into the.
Sep 02, (Market Insight Reports) -- Interleukin-23 (IL-23) Inhibitor – Pipeline Insight, report by. Antibodies that inhibit IL-12/23 or IL-23 are key treatment options for patients with psoriasis. An additional consideration when selecting the therapeutic regimen for psoriasis is the required frequency of dosing, which is an aspect in which IL-17 and IL-23 inhibitors differ.
Digestive Disease Week, San Diego, USA, 21–24th May 16. Due to the pathophysiology of the disease, there is a rationale for using multiple classes of biologics. The approval is based on two pivotal P-III clinical trials (DISCOVER-1 and DISCOVER-2) assessing Tremfya (100 mg, SC, q8w) following two starter doses @0 & 4wks.
The advent of IL-23 inhibitors is an exciting advance in the treatment of psoriasis, Dr Blauvelt and other experts said here at the 26th European Academy of Dermatology and Venereology (EADV. Still, IL-23/23 inhibitors were associated with a lower risk of serious infections compared with TNF inhibitors (HR = 0.59;. Conclusions IL-12 and IL-23 inhibitors remain on the forefront of treatment options for inflammatory diseases such as psoriasis, Crohn’s disease, multiple sclerosis, and rheumatoid arthritis.
Interleukin inhibitors are immunosuppressive agents which inhibit the action of interleukins. A detailed picture of the Interleukin-23 (IL-23) Inhibitor pipeline landscape is provided, which includes the topic overview and Interleukin-23 (IL-23) Inhibitor mechanism of action. Interleukin (IL)‐17, IL‐12/23, and IL‐23 inhibitors are associated with low infection risk, with IL‐17 and IL‐23 favored over IL‐12/23 inhibitors.
Given the years of clinical experience with TNF antagonists, PDE4 antagonists, and, more recently, IL-17 agents, it is not likely that targeted IL-23 inhibitors will be recommended as first-line thera- py for several reasons. Interleukins are a group of cytokines which are synthesized by lymphocytes, monocytes, macrophages, and certain other cells. It was studied in a 24-week trial involving 159 biologic-naïve, active AS patients.
Scope of the Report. Interleukin 23 (IL-23) Inhibitors Ilumya (tildrakizumab-asmn), Skyrizi (risankizumab-rzaa) and Tremfya (guselkumab) work by targeting interleukin 23 (IL-23). IL-23 inhibitors will likely not be an exception to this trend.
View Reply to Parvu and Parvu. Click image to enlarge. Results of a multicentre, double-blind, phase 2, randomised, controlled study Previous Article Closed-loop insulin delivery in suboptimally controlled type 1 diabetes:.
The MarketWatch News Department was not involved in the creation of this content. IL-23 inhibitors are among the many medications that doctors can use to treat moderate-to-severe psoriasis. Ilumya, Skyrizi and Tremfya work to reduce psoriatic symptoms and slow disease progression.
We continue our series, Therapeutic Cheat Sheet, with a closer look at IL-23 inhibitors. IL-23 has been recognized as a major factor in the etiology and pathogenesis of psoriasis, and recent therapeutic development has focused on inhibition of the inflammatory cytokine. A functional receptor for IL-23 (the IL-23 receptor) has been identified and is composed of IL-12R β1 and IL-23R.
Herein, we describe the treatment of 2 patients with suberythrodermic pityriasis rubra pilaris (PRP) with guselkumab, a specific interleukin 23 (IL-23)p19 inhibitor. IL-23, a cytokine involved in inflammatory processes, is thought to be linked to a number of chronic immune-mediated diseases, including psoriasis. Pivotal trials and postmarketing data also suggest that IL‐17 and IL‐23 blockers are safer than tumor necrosis factor alpha blockers.
Risankizumab selectively inhibits interleukin-23 (IL-23), a key inflammatory protein, by binding to its p19 subunit. Interleukin 23 is a heterodimeric cytokine composed of two subunits:. At this time, 2 inhibitors of IL-23 p19 have been approved by the United States Food and Drug Administration, guselkumab and tildrakizumab.
While IL-17 inhibitors require dosing every 2–4 weeks, 32,33,44 IL-12/23 and IL-23 inhibitors are dosed less frequently, typically every 8–12 weeks. IL-12 and IL-23 inhibitors remain on the forefront of treatment options for inflammatory diseases such as psoriasis, Crohn’s disease, multiple sclerosis, and rheumatoid arthritis. A study of the IL-23 risankizumab in active ankylosing spondylitis (AS) patients failed to show efficacy and did not meet primary efficacy endpoints in a 6-month trial.Risankizumab (RIZ) is a new, humanised anti-IL-23 monoclonal antibody that targets the p19 subunit of interleukin-23 (IL-23).
9,10 Clinical trials for psoriasis treatments have successfully used monoclonal antibodies against IL-17 and IL-23, supporting the current evidence of these cytokines as key drivers of psoriasis. Most people who take IL-23 inhibitors experience few side effects. There are robust immunological and, importantly, functional differences between these cytokines.
“Tremfya is the first and only selective IL-23 inhibitor approved for both active psoriatic arthritis and moderate to severe plaque psoriasis, as well as the only biologic approved for the. ^2 Psoriatic arthritis is an inflammatory form of arthritis affecting approximately 94,000 Canadians. Recovery from COVID-19 in a patient with spondyloarthritis treated with TNF-alpha inhibitor etanercept.
Click here to read more about IL-23 inhibitors. Two other agents, risankizumab and mirikizumab, have completed phase 3 and phase 2 of development, respectively. The Phase II trial compared the efficacy of risankizumab to that of ustekinumab, an IL-12 and IL-23 inhibitor, in patients with moderate-to-severe plaque psoriasis.
B Feagan et al. These inhibitors can then effectively block the protein from carrying out its function. The drug is administered at a dose of 150 mg, in two subcutaneous injections, every 12 weeks, after starting doses at weeks 0 and 4.
Results of a randomized, double-blind, placebo-controlled Phase II study. This symposium gave an overview of the current treatment landscape for psoriasis, clinical developments, and recent clinical trials. Boehringer says anti-IL-23 drug beats Stelara in psoriasis trial Interleukin-23 inhibitor outperforms Johnson & Johnson's blockbuster New data from a phase II trial of Boehringer Ingelheim's psoriasis candidate BI back up earlier results showing it is more effective than a rival drug from Johnson & Johnson.
Frontiers The Interleukin 23 Interleukin 17 Axis Links Adaptive And Innate Immunity In Psoriasis Immunology
Il 12 Il 23 And Il 17 In Ibd Immunobiology And Therapeutic Targeting Nature Reviews Gastroenterology Hepatology

New Biologics And Small Molecules Under Development For The Treatment Download Scientific Diagram
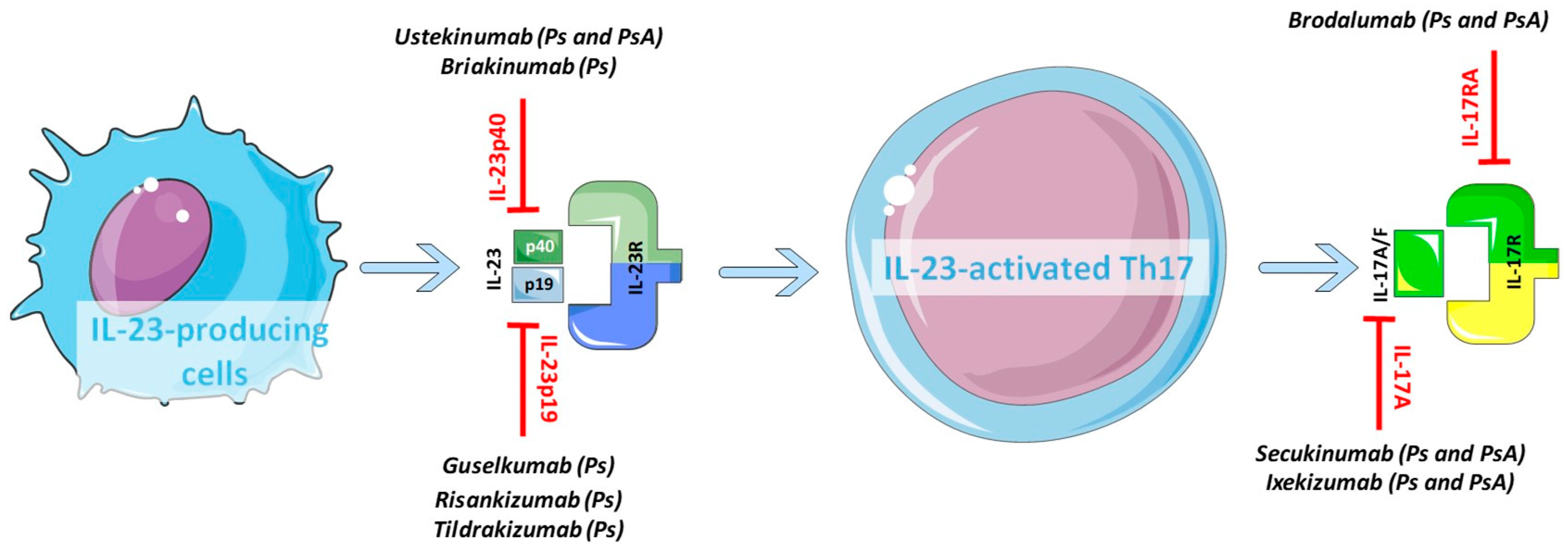
Dr Hanady Manasfi Key Difference In Mechanism Of Action Of Il 17 And Il 23 Inhibitors In Psoriasis

Insights Into Il 23 Biology From Structure To Function Sciencedirect

Irak1 4 Inhibitor Rigel Pharmaceuticals
Apilimod Sta 5326 Il 12 Il 23 Inhibitor Medchemexpress
New Biologics In Psoriasis An Update On Il 23 And Il 17 Inhibitors Mdedge Dermatology

Risankizumab An Il 23 Inhibitor For Ankylosing Spondylitis Results Of A Randomised Double Blind Placebo Controlled Proof Of Concept Dose Finding Phase 2 Study Annals Of The Rheumatic Diseases
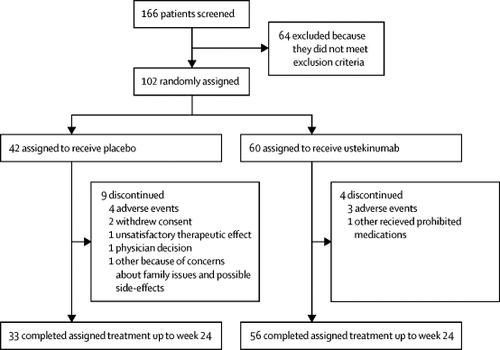
Efficacy And Safety Of Ustekinumab An Il 12 And Il 23 Inhibitor In Patients With Active Systemic Lupus Erythematosus Results Of A Multicentre Double Blind Phase 2 Randomised Controlled Study Lancet X Mol

Risankizumab Versus Ustekinumab For Moderate To Severe Plaque Psoriasis Nejm
Selective Interleukin 23 P19 Inhibition Another Game Changer In Psoriasis Focus On Risankizumab Springerlink
Assessing The Relative Efficacy Of Interleukin 17 And Interleukin 23 Targeted Treatments For Moderate To Severe Plaque Psoriasis A Systematic Review And Network Meta Analysis Of Pasi Response

Discovery Of The Il 23 Il 17 Signaling Pathway And The Treatment Of Psoriasis The Journal Of Immunology

Biologic Medication Information Crutchfield Dermatology

The Interleukin Il 23 Il 17 Axis In Ankylosing Spondylitis New Advances And Potentials For Treatment Jethwa 16 Clinical Amp Experimental Immunology Wiley Online Library
Psoriasis Archives Maui Derm
Il 23 Signaling Regulation Of Pro Inflammatory T Cell Migration Uncovered By Phosphoproteomics

Johnson Johnson Sugarcone Biotech
Understanding How Il 23p19 Inhibitors Work In Psoriasis Ppt Download

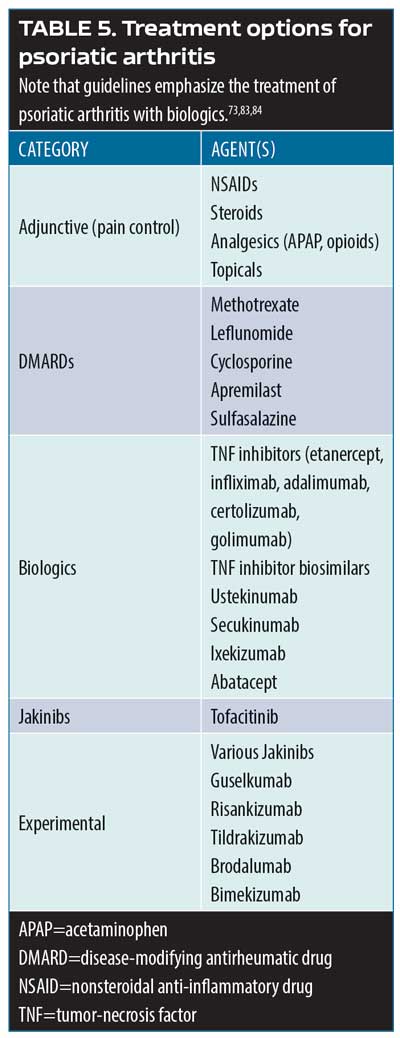
Effective Management Of Psoriatic Arthritis Tailoring Treatments Transcript
Tyk2 Inhibition By A Novel Small Molecule Blocks Il 23 Induced Stat3 Download Scientific Diagram

Discovery Of The Il 23 Il 17 Signaling Pathway And The Treatment Of Psoriasis The Journal Of Immunology

Pdf Dual Inhibition Of Interleukin 23 And Interleukin 17 Offers Superior Efficacy In Mouse Models Of Autoimmunity Semantic Scholar

Efficacy And Safety Of Ustekinumab An Il 12 And Il 23 Inhibitor In Patients With Active Systemic Lupus Erythematosus Results Of A Multicentre Double Blind Phase 2 Randomised Controlled Study The Lancet
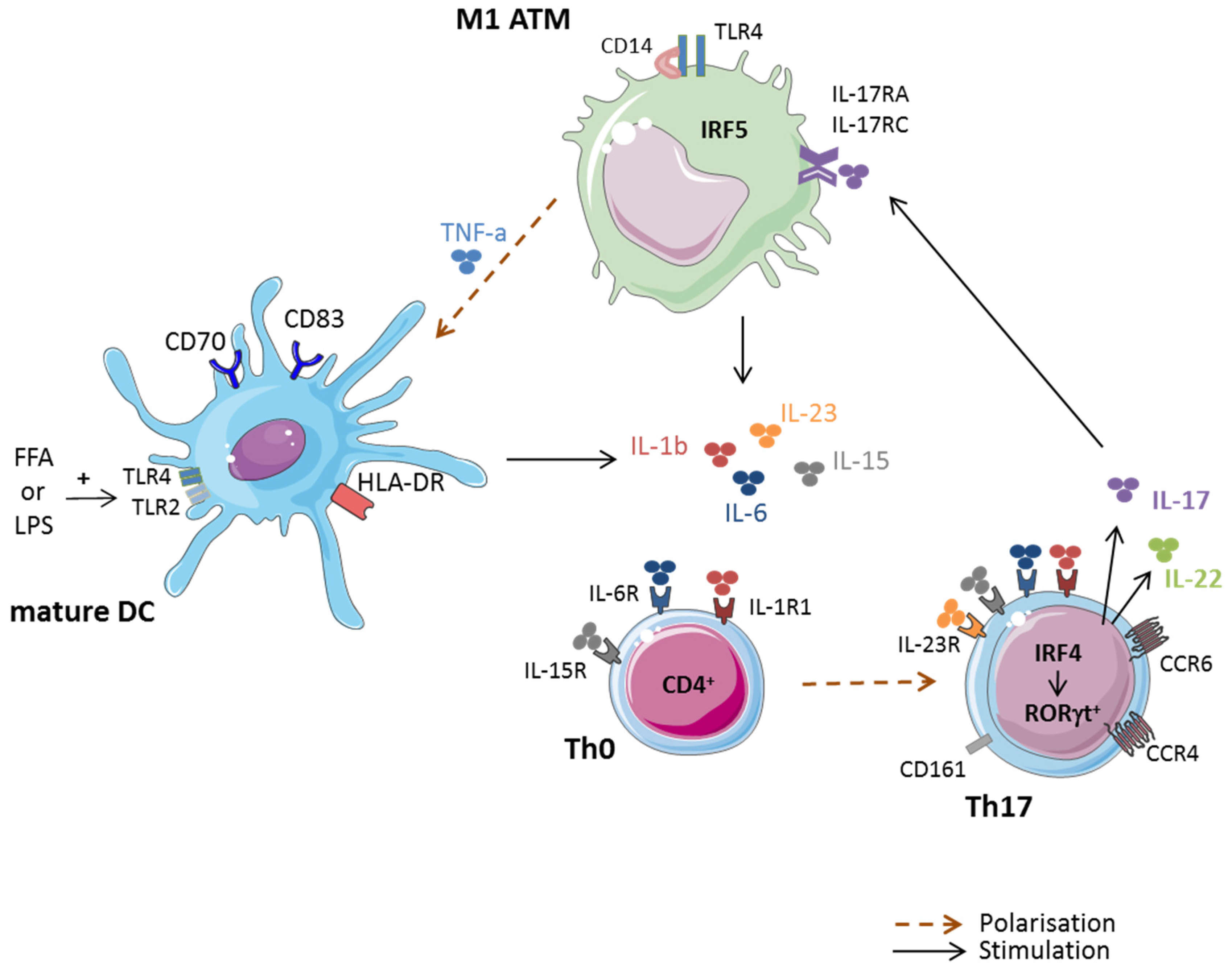
Jcm Free Full Text Pathogenic Role Of Il 17 Producing Immune Cells In Obesity And Related Inflammatory Diseases Html

Fda Approves Guselkumab For Psoriatic Arthritis
Q Tbn 3aand9gcsubza9lbaazzjlfe0lzbzd7d4q3rv4yk4iafrwys97pdyc Xgt Usqp Cau
Apilimod Inhibits The Production Of Il 12 And Il 23 And Reduces Dendritic Cell Infiltration In Psoriasis

Interleukin 23 Production In Dendritic Cells Is Negatively Regulated By Protein Phosphatase 2a Pnas

Applying Science To Improve The Individualized Treatment Of Patients With Psoriasis Abrar Qureshi Md Mph Chief Of The Department Of Dermatology Rhode Ppt Download
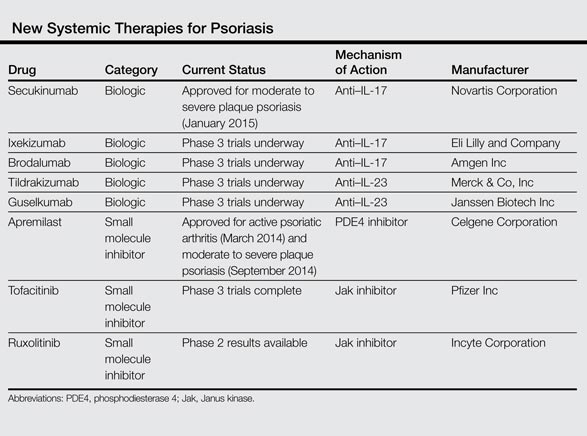
New Systemic Therapies For Psoriasis Mdedge Dermatology

A Folding Switch Regulates Interleukin 27 Biogenesis And Secretion Of Its A Subunit As A Cytokine Pnas

The Biologic Revolution In Treatment Of Psoriasis And Psa The Dermatologist
Q Tbn 3aand9gcsfn9vivvghj26pt6urbrffs4cxx Vaudilv9mpgutftirfti Usqp Cau

Why Did Il 23p19 Inhibition Fail In As A Tale Of Tissues Trials Or Translation Annals Of The Rheumatic Diseases

Paradoxical Gastrointestinal Effects Of Interleukin 17 Blockers Annals Of The Rheumatic Diseases

A Cytokine Network Involving Il 36g Il 23 And Il 22 Promotes Antimicrobial Defense And Recovery From Intestinal Barrier Damage Pnas
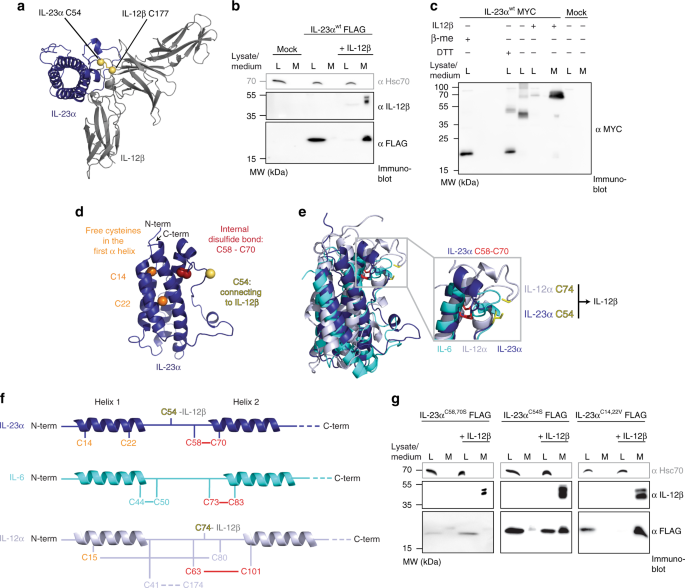
The Molecular Basis Of Chaperone Mediated Interleukin 23 Assembly Control Nature Communications
Q Tbn 3aand9gcr3mhz6mcjy6jek4cw2jcumiwk Oyqmbe3r74bs4ois7cvvf9 O Usqp Cau
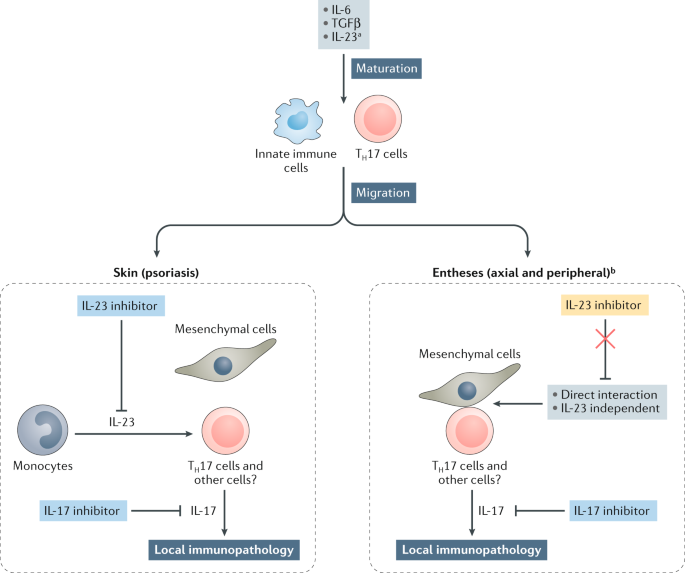
The Il 23 Il 17 Pathway As A Therapeutic Target In Axial Spondyloarthritis Nature Reviews Rheumatology
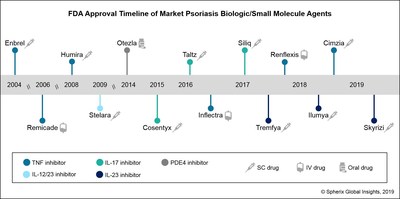
Robust Use Of Janssen S Tremfya And Positive Early Launch Metrics For Abbvie S Skyrizi May Threaten Growth Of Il 17 Inhibitors In Us Psoriasis Market According To Spherix Global Insights Markets Insider
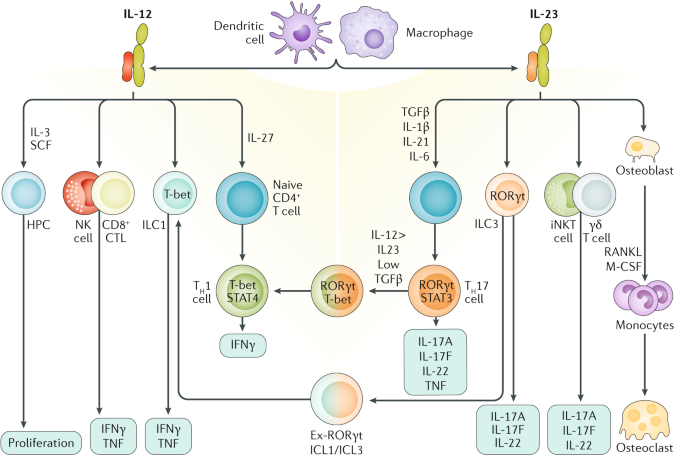
Il 12 Il 23 And Il 17 In Ibd Immunobiology And Therapeutic Targeting Nature Reviews Gastroenterology Hepatology
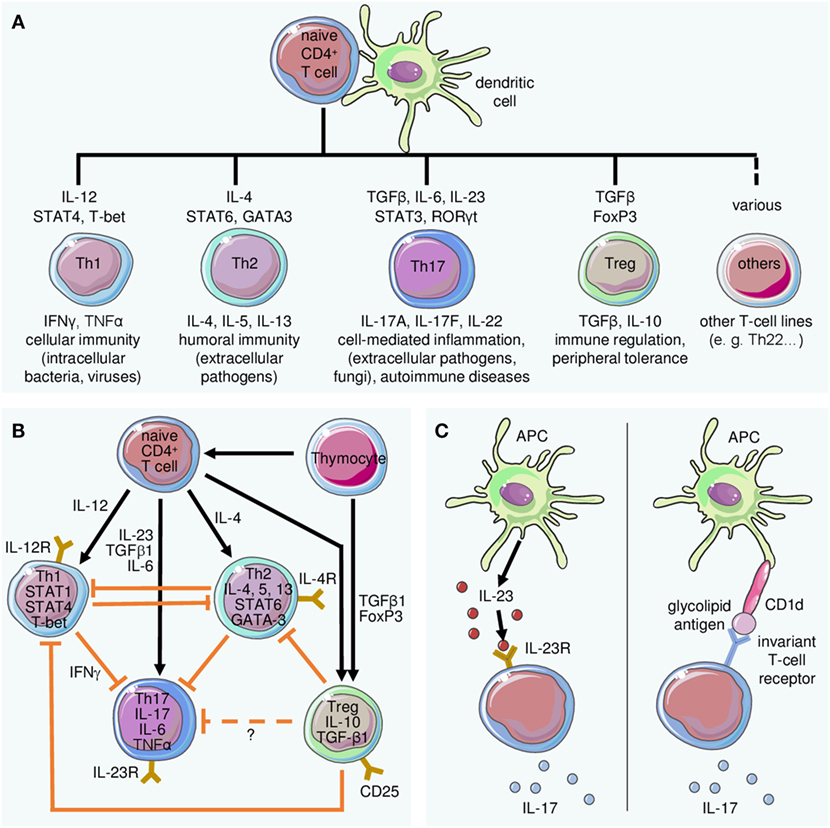
Frontiers The Interleukin 23 Interleukin 17 Axis Links Adaptive And Innate Immunity In Psoriasis Immunology
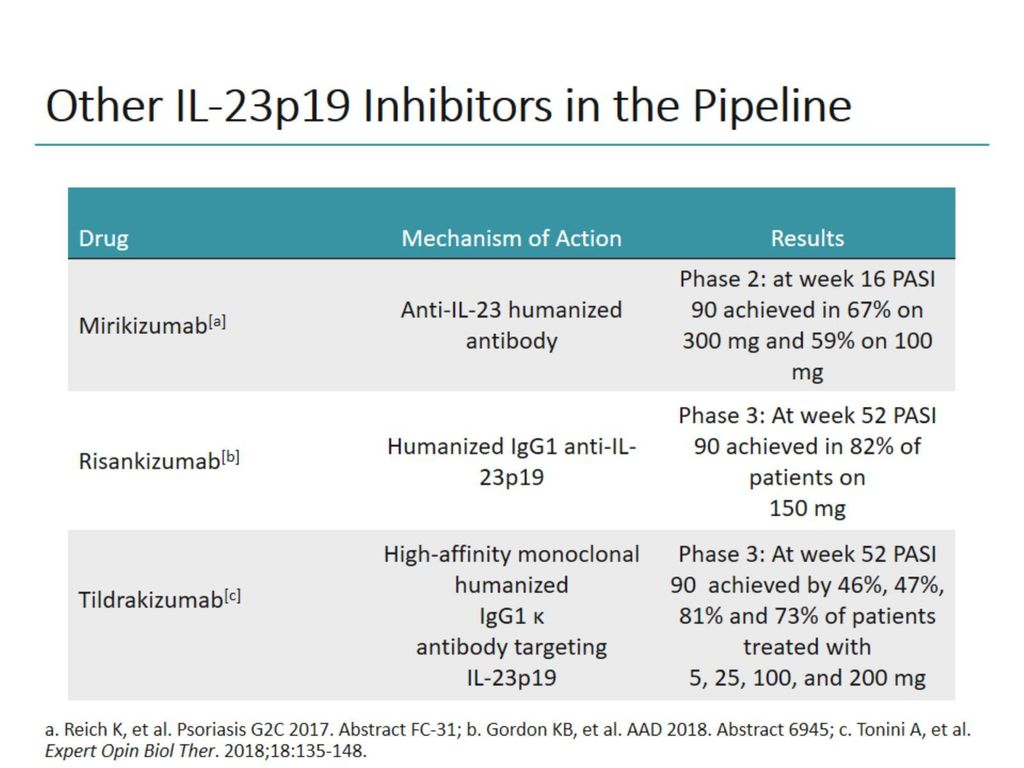
Evolution Of Treatment Strategies Targeting Il 23 For Psoriasis Ppt Download

Classes Of Biologics For The Treatment Of Ibd Alpco

Psoriasis Pathogenesis And The Development Of Novel Targeted Immune Therapies Journal Of Allergy And Clinical Immunology
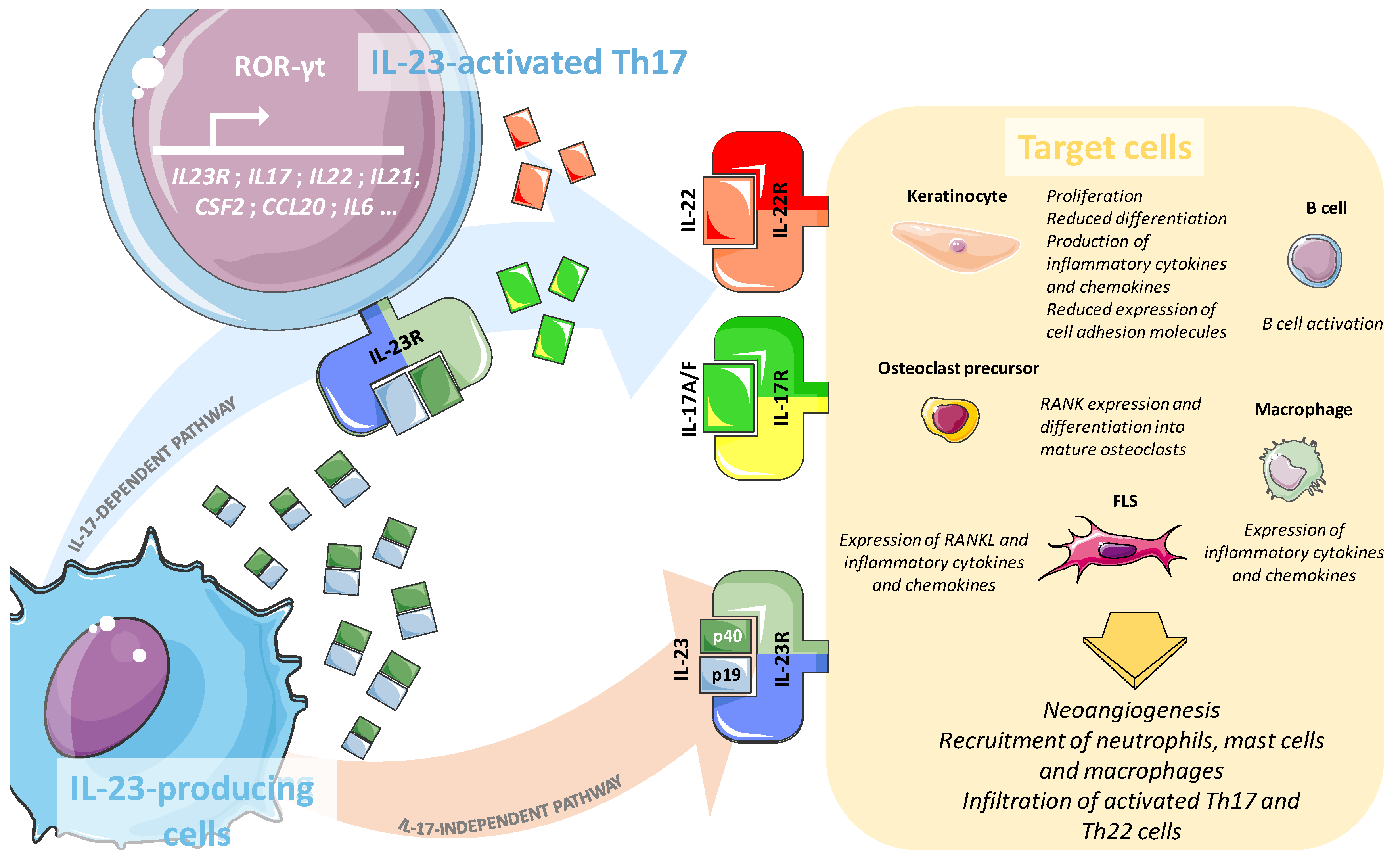
Ijms Free Full Text Role Of The Il 23 Il 17 Axis In Psoriasis And Psoriatic Arthritis The Clinical Importance Of Its Divergence In Skin And Joints Html
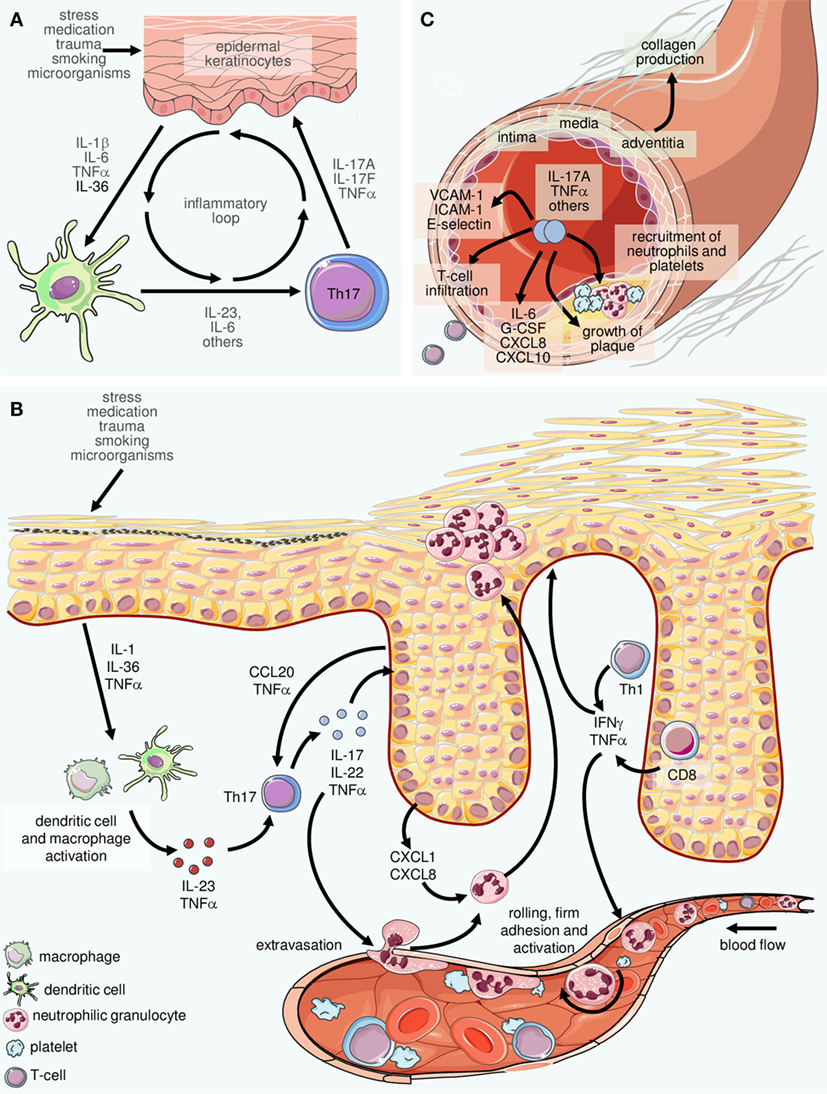
Frontiers The Interleukin 23 Interleukin 17 Axis Links Adaptive And Innate Immunity In Psoriasis Immunology

Pdf Risankizumab An Il 23 Inhibitor For Ankylosing Spondylitis Results Of A Randomised Double Blind Placebo Controlled Proof Of Concept Dose Finding Phase 2 Study
2

Interleukins From Il 1 To Il 38 Interferons Transforming Growth Factor B And Tnf A Receptors Functions And Roles In Diseases Journal Of Allergy And Clinical Immunology

Pdf A Review Of Guselkumab An Il 23 Inhibitor For Moderate To Severe Plaque Psoriasis
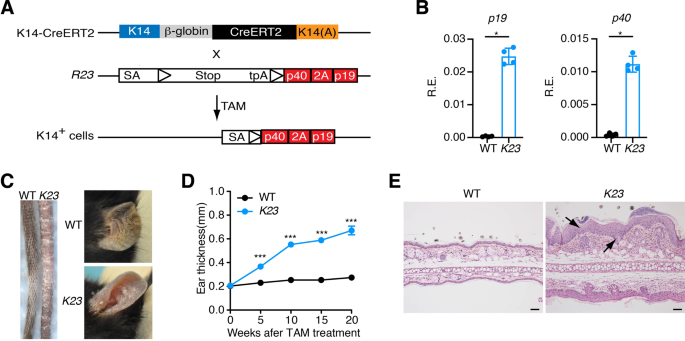
Skin Expression Of Il 23 Drives The Development Of Psoriasis And Psoriatic Arthritis In Mice Scientific Reports

Biologic Therapies For Psoriasis Plastic Surgery Key

Biologics In Psoriasis The Next Generation Practical Dermatology
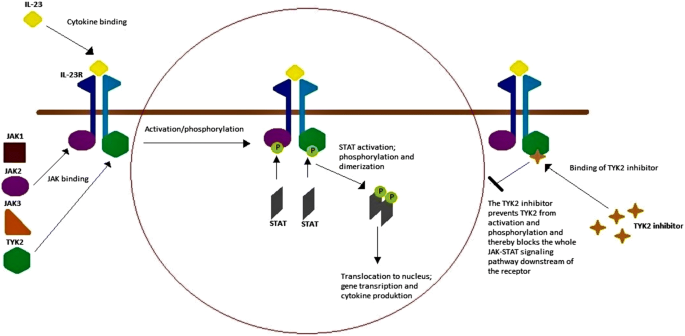
Systemic Treatment Of Psoriasis With Jak Inhibitors A Review Springerlink

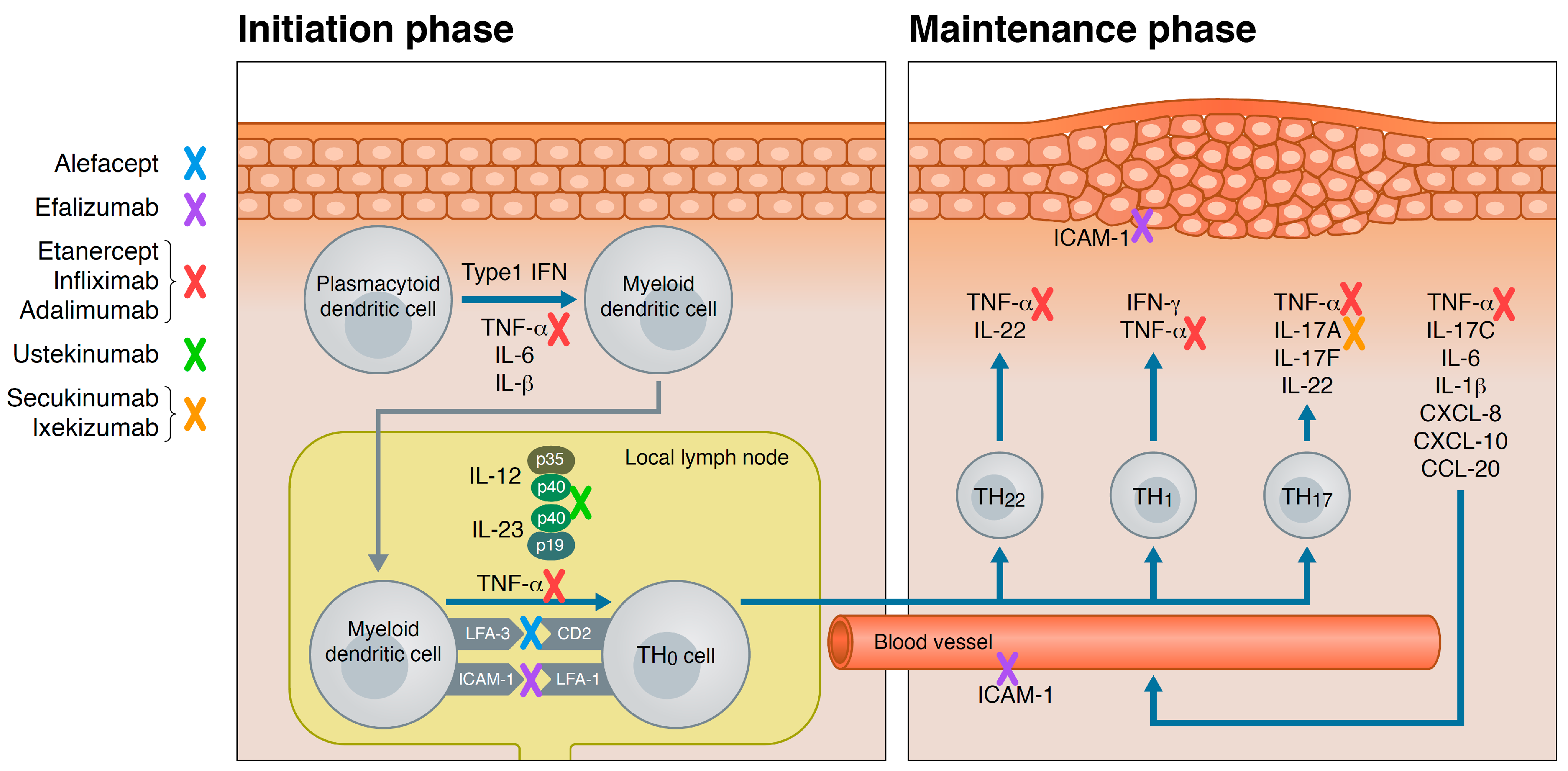
Potential Clinical Implications Of The Latest Data On Approved And Em

Tildrakizumab For The Treatment Of Psoriasis Immunotherapy
The Il 23 Il 17 Immune Axis From Mechanisms To Therapeutic Testing Nature Reviews Immunology
Ijms Free Full Text Old And New Biological Therapies For Psoriasis Html
Risk For Development Of Inflammatory Bowel Disease Under Inhibition Of Interleukin 17 A Systematic Review And Meta Analysis
2

Interleukin 23 Il 23 Inhibitor Pipeline Insight

Full Text Nail Psoriasis Clinical Features Pathogenesis Differential Diagnose Ptt

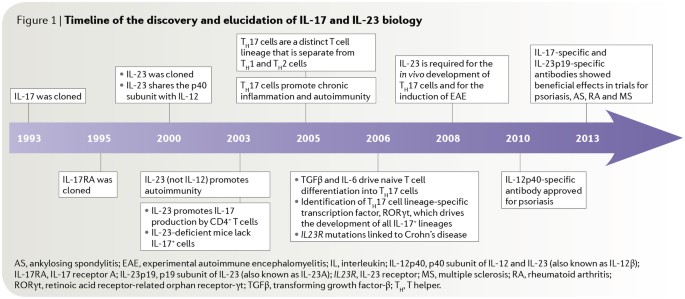
Historical Timeline Of Discoveries And Evolving Pathophysiologic Download Scientific Diagram

Guselkumab Versus Secukinumab For The Treatment Of Moderate To Severe Psoriasis Eclipse Results From A Phase 3 Randomised Controlled Trial The Lancet

The Il 23 T17 Pathogenic Axis In Psoriasis Is Amplified By Keratinocyte Responses Sciencedirect

Tofacitinib Attenuates Pathologic Immune Pathways In Patients With Psoriasis A Randomized Phase 2 Study Journal Of Allergy And Clinical Immunology

A Receptor For The Heterodimeric Cytokine Il 23 Is Composed Of Il 12rb1 And A Novel Cytokine Receptor Subunit Il 23r The Journal Of Immunology

Psoriasis Treatment Unmet Needs Present 19 Opportunities Pm360

Efficacy And Safety Of Medi70 An Antibody Against Interleukin 23 In Patients With Moderate To Severe Crohn S Disease A Phase 2a Study Gastroenterology
Il 23 Signaling Regulation Of Pro Inflammatory T Cell Migration Uncovered By Phosphoproteomics

Stat3 And Nf Kb Signal Pathway Is Required For Il 23 Mediated Il 17 Production In Spontaneous Arthritis Animal Model Il 1 Receptor Antagonist Deficient Mice The Journal Of Immunology
Ijms Free Full Text Role Of The Il 23 Il 17 Axis In Psoriasis And Psoriatic Arthritis The Clinical Importance Of Its Divergence In Skin And Joints Html

Pikfyve A Class Iii Pi Kinase Is The Target Of The Small Molecular Il 12 Il 23 Inhibitor Apilimod And A Player In Toll Like Receptor Signaling Sciencedirect
Q Tbn 3aand9gctknda Sihqkqm Fisk5pqk4a5nb Irkeweixmpoesa8 Yqxxu Usqp Cau

The Role Of Il 23 And The Il 23 Th17 Immune Axis In The Pathogenesis And Treatment Of Psoriasis Girolomoni 17 Journal Of The European Academy Of Dermatology And Venereology Wiley Online Library

Psoriasis And Psa Beyond Skin And Joint Involvement The Dermatologist

Biologic Therapy For Psoriasis Still Searching For The Best Target
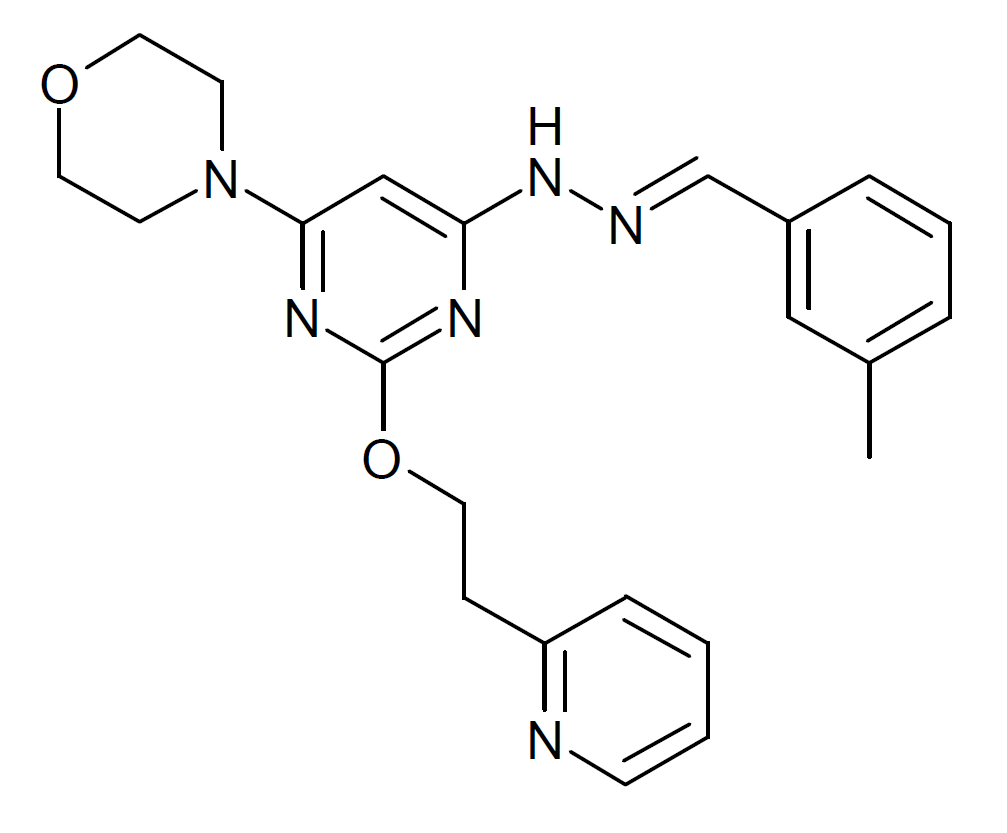
Apilimod Wikipedia

Ustekinumab In The Treatment Of Psoriasis And Psoriatic Arthritis Topic Of Research Paper In Clinical Medicine Download Scholarly Article Pdf And Read For Free On Cyberleninka Open Science Hub

Il 23 In Inflammatory Bowel Diseases And Colon Cancer Sciencedirect

New Phase 3 Data For Tremfya Guselkumab A First In Class Il 23 P19 Subunit Inhibitor Show Consistent High Levels Of Skin Clearance Through Four Years In Adult Patients With Moderate To Severe Plaque Psoriasis
Ijms Free Full Text Molecular Mechanisms And Management Of A Cutaneous Inflammatory Disorder Psoriasis Html
Il 12 And Il 23 Cytokines From Discovery To Targeted Therapies For Immune Mediated Inflammatory Diseases Nature Medicine
Dosing Administration Ilumya Tildrakizumab Asmn Hcp

Il 23 Inhibitors For Treating Psoriasis What To Know

Putting Together The Psoriasis Puzzle An Update On Developing Targeted Therapies Disease Models Mechanisms

Why Did Il 23p19 Inhibition Fail In As A Tale Of Tissues Trials Or Translation Annals Of The Rheumatic Diseases

Apremilast Wikipedia

The Biologic Revolution In Treatment Of Psoriasis And Psa The Dermatologist

Figure 1 From Review Of Ustekinumab An Interleukin 12 And Interleukin 23 Inhibitor Used For The Treatment Of Plaque Psoriasis Semantic Scholar

Jci Tyk2 Inhibition Reduces Type 3 Immunity And Modifies Disease Progression In Murine Spondyloarthritis

Structure Activity Relationship Study Target Identification And Pharmacological Characterization Of A Small Molecular Il 12 23 Inhibitor Apy01 Sciencedirect
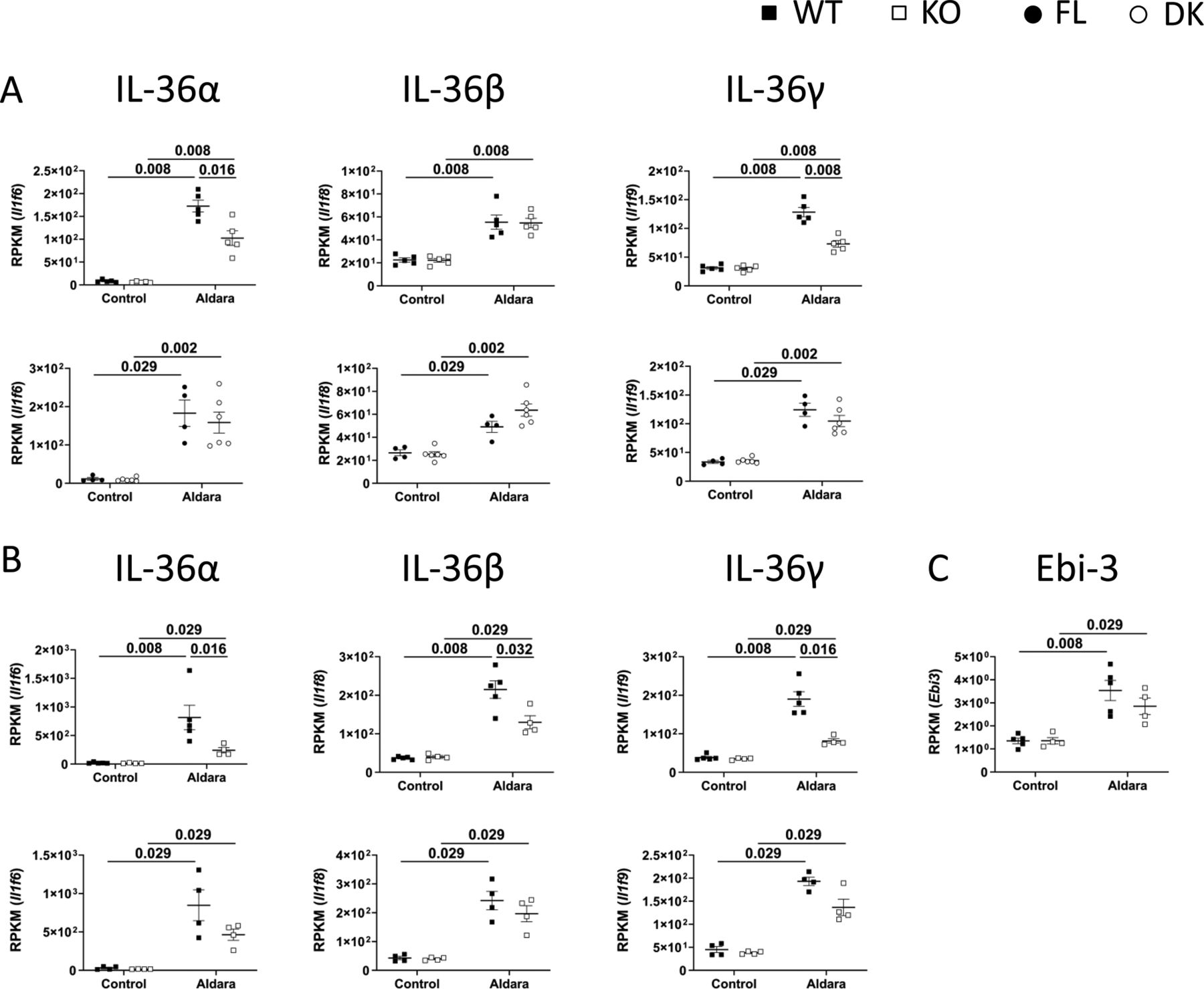
Il 36 Signaling In Keratinocytes Controls Early Il 23 Production In Psoriasis Like Dermatitis Life Science Alliance

Fda Approves Tremfya First Il 23 Inhibitor For Psoriatic Arthritis

Il 12 Il 23 And Il 17 In Ibd Immunobiology And Therapeutic Targeting Semantic Scholar



